“Unraveling the Legal Labyrinth: Can AI Revolutionize Pharma Without Copyright Catastrophes?”
To ensure AI is used safely, securely, and compliantly, pharma and clinical research organizations must collaborate to address copyright and data-sharing challenges — or risk stalling innovation. To explore these challenges, we brought together experts in the field from the Alliance’s membership to highlight key risks and outline best practices for responsible AI adoption.
Why Copyright Compliance Is Critical
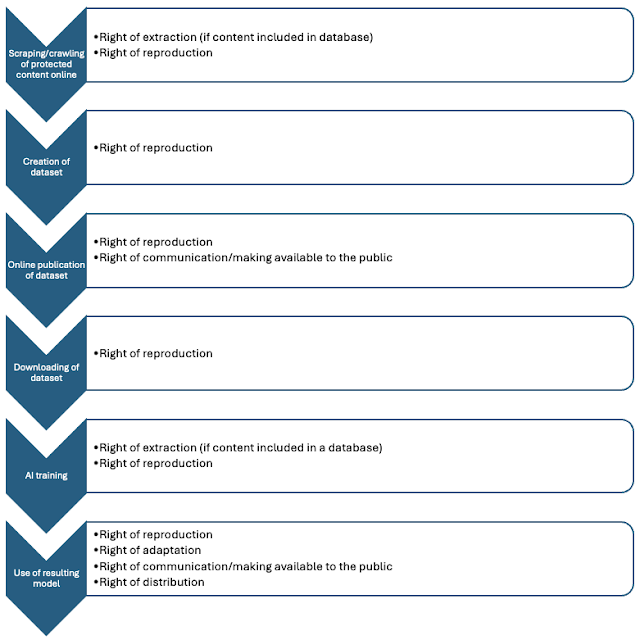
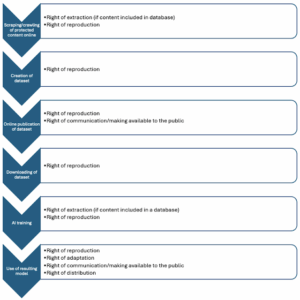
AI models are trained on a range of scientific data sources. Dr. Babis Marmanis, who holds a Ph.D. in applied mathematics and scientific computing and serves as chief technology officer at the Copyright Clearance Center, explains, “Generative AI outcomes improve when trained on responsibly sourced, copyrighted works. The training and fine-tuning of AI models in pharma R&D includes high-value scientific literature, and that use entails making and storing copies of these documents. In many cases, with the appropriate prompts, portions or the entirety of the original works can be recalled by end users and derivative or very similar outputs can be created. Without appropriate licenses, this creates copyright infringement risks.”